Nápady 115+ Atom Vs Ion Diagram Čerstvé
Nápady 115+ Atom Vs Ion Diagram Čerstvé. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Neutral atoms can be turned into positively charged ions by.
Prezentováno Difference Between Atom And Ion Difference Between
Jun 30, 2011 · the key difference between atom and ion is the charge. Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. Aug 15, 2020 · aug 15, 2020 · bohr diagrams. Producing ions is a way to achieve the noble gas configuration and thus become stable.Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms.
Apr 09, 2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. The single elements are hardly stable under natural conditions. They can be either positively charged ions or negatively charged ions. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. They form various combinations between them or with other elements in order to exist.

Atoms are the small building blocks of all existing substances. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Jun 30, 2011 · the key difference between atom and ion is the charge. Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions. Aug 15, 2020 · aug 15, 2020 · bohr diagrams.. Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion.

Atom is electrically neutral while ion is electrically charged. Jun 30, 2011 · the key difference between atom and ion is the charge. The single elements are hardly stable under natural conditions. The single elements are hardly stable under natural conditions.

Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. If an atom is charged electrically it is considered an ion. If an atom contains maximum number of electrons than protons, then it is called anion. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Producing ions is a way to achieve the noble gas configuration and thus become stable. Apr 09, 2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. They can be either positively charged ions or negatively charged ions.

Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. An ion with more protons than electrons carries a net positive charge and is called a cation. The single elements are hardly stable under natural conditions. Atom is electrically neutral while ion is electrically charged. They can be either positively charged ions or negatively charged ions. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different.. They can be either positively charged ions or negatively charged ions.

Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Atoms are the small building blocks of all existing substances. Atom is electrically neutral while ion is electrically charged.

An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different.. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. When an ion is formed, the number of protons does not change. An ion with more electrons than protons carries a … They can be either positively charged ions or negatively charged ions. An ion with more protons than electrons carries a net positive charge and is called a cation. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion. Atoms are the small building blocks of all existing substances. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Atom is electrically neutral while ion is electrically charged. If an atom contains maximum number of electrons than protons, then it is called anion.

Feb 02, 2020 · atoms are composed of equal protons and electrons as in their existing natural forms as a whole with varying numbers of electrons and protons with varying atomic numbers. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. When an ion is formed, the number of protons does not change... Jun 30, 2011 · the key difference between atom and ion is the charge.

Mar 17, 2012 · mar 17, 2012 · atom vs ion... If an atom contains maximum number of electrons than protons, then it is called anion.

Aug 15, 2020 · aug 15, 2020 · bohr diagrams... Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. When an ion is formed, the number of protons does not change. An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. An ion with more electrons than protons carries a … Mar 17, 2012 · mar 17, 2012 · atom vs ion. Producing ions is a way to achieve the noble gas configuration and thus become stable. Apr 09, 2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Feb 02, 2020 · atoms are composed of equal protons and electrons as in their existing natural forms as a whole with varying numbers of electrons and protons with varying atomic numbers. Aug 15, 2020 · aug 15, 2020 · bohr diagrams. Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions... Neutral atoms can be turned into positively charged ions by.

They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion... Neutral atoms can be turned into positively charged ions by. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Jun 30, 2011 · the key difference between atom and ion is the charge. Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion. Mar 17, 2012 · mar 17, 2012 · atom vs ion.. Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions.

Mar 17, 2012 · mar 17, 2012 · atom vs ion... Jun 30, 2011 · the key difference between atom and ion is the charge. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. An ion with more electrons than protons carries a … Aug 15, 2020 · aug 15, 2020 · bohr diagrams. Producing ions is a way to achieve the noble gas configuration and thus become stable. An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. They form various combinations between them or with other elements in order to exist. Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. Mar 17, 2012 · mar 17, 2012 · atom vs ion.. Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions.

Producing ions is a way to achieve the noble gas configuration and thus become stable. An ion with more protons than electrons carries a net positive charge and is called a cation. The single elements are hardly stable under natural conditions. Apr 09, 2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions. Producing ions is a way to achieve the noble gas configuration and thus become stable. Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion.

If an atom contains maximum number of electrons than protons, then it is called anion. An ion with more electrons than protons carries a … Feb 02, 2020 · atoms are composed of equal protons and electrons as in their existing natural forms as a whole with varying numbers of electrons and protons with varying atomic numbers. The single elements are hardly stable under natural conditions. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. When an ion is formed, the number of protons does not change. Atoms are the small building blocks of all existing substances. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. If an atom contains maximum number of electrons than protons, then it is called anion. The single elements are hardly stable under natural conditions.

An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. They form various combinations between them or with other elements in order to exist. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion. An ion with more electrons than protons carries a … Apr 09, 2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Mar 17, 2012 · mar 17, 2012 · atom vs ion. They can be either positively charged ions or negatively charged ions.

Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms... .. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms.

Mar 17, 2012 · mar 17, 2012 · atom vs ion. Feb 02, 2020 · atoms are composed of equal protons and electrons as in their existing natural forms as a whole with varying numbers of electrons and protons with varying atomic numbers. Atom is electrically neutral while ion is electrically charged. Aug 15, 2020 · aug 15, 2020 · bohr diagrams. Atoms are the small building blocks of all existing substances.. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun.

Mar 17, 2012 · mar 17, 2012 · atom vs ion. Aug 15, 2020 · aug 15, 2020 · bohr diagrams.
An ion with more protons than electrons carries a net positive charge and is called a cation. Producing ions is a way to achieve the noble gas configuration and thus become stable. If an atom is charged electrically it is considered an ion. Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. Atoms are the small building blocks of all existing substances. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Neutral atoms can be turned into positively charged ions by. Aug 15, 2020 · aug 15, 2020 · bohr diagrams. When an ion is formed, the number of protons does not change. Atom is electrically neutral while ion is electrically charged. Apr 09, 2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.. Neutral atoms can be turned into positively charged ions by.

Feb 02, 2020 · atoms are composed of equal protons and electrons as in their existing natural forms as a whole with varying numbers of electrons and protons with varying atomic numbers. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. They form various combinations between them or with other elements in order to exist. Atoms are the small building blocks of all existing substances. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. When an ion is formed, the number of protons does not change. Mar 17, 2012 · mar 17, 2012 · atom vs ion.

Atom is electrically neutral while ion is electrically charged... An ion with more electrons than protons carries a …

They form various combinations between them or with other elements in order to exist. Feb 02, 2020 · atoms are composed of equal protons and electrons as in their existing natural forms as a whole with varying numbers of electrons and protons with varying atomic numbers. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion. Mar 17, 2012 · mar 17, 2012 · atom vs ion. Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Atom is electrically neutral while ion is electrically charged. They form various combinations between them or with other elements in order to exist. When an ion is formed, the number of protons does not change.

In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Feb 02, 2020 · atoms are composed of equal protons and electrons as in their existing natural forms as a whole with varying numbers of electrons and protons with varying atomic numbers. Apr 09, 2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. They can be either positively charged ions or negatively charged ions. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. An ion with more protons than electrons carries a net positive charge and is called a cation.

Apr 09, 2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators... Atom is electrically neutral while ion is electrically charged. An ion with more protons than electrons carries a net positive charge and is called a cation. Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. If an atom is charged electrically it is considered an ion. If an atom contains maximum number of electrons than protons, then it is called anion. Mar 17, 2012 · mar 17, 2012 · atom vs ion. They can be either positively charged ions or negatively charged ions. They form various combinations between them or with other elements in order to exist. Aug 15, 2020 · aug 15, 2020 · bohr diagrams.

They form various combinations between them or with other elements in order to exist. Neutral atoms can be turned into positively charged ions by. They can be either positively charged ions or negatively charged ions.. Feb 02, 2020 · atoms are composed of equal protons and electrons as in their existing natural forms as a whole with varying numbers of electrons and protons with varying atomic numbers.

Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions. Jun 30, 2011 · the key difference between atom and ion is the charge. When an ion is formed, the number of protons does not change. Apr 09, 2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Atoms are the small building blocks of all existing substances. They can be either positively charged ions or negatively charged ions. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. They can be either positively charged ions or negatively charged ions.

Apr 09, 2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.. If an atom contains maximum number of electrons than protons, then it is called anion. Atoms are the small building blocks of all existing substances... Producing ions is a way to achieve the noble gas configuration and thus become stable.
/cation-and-an-anion-differences-606111-v2_preview-5b44daf9c9e77c0037679d52.png)
An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. Atom is electrically neutral while ion is electrically charged. Apr 09, 2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different.. If an atom is charged electrically it is considered an ion.

Atom is electrically neutral while ion is electrically charged. Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion. Neutral atoms can be turned into positively charged ions by. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Jun 30, 2011 · the key difference between atom and ion is the charge.. Jun 30, 2011 · the key difference between atom and ion is the charge.

In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. When an ion is formed, the number of protons does not change. Atom is electrically neutral while ion is electrically charged. Atoms are the small building blocks of all existing substances. If an atom is charged electrically it is considered an ion. If an atom contains maximum number of electrons than protons, then it is called anion. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. An ion with more protons than electrons carries a net positive charge and is called a cation. Jun 30, 2011 · the key difference between atom and ion is the charge.. Producing ions is a way to achieve the noble gas configuration and thus become stable.

When an ion is formed, the number of protons does not change. When an ion is formed, the number of protons does not change. Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. If an atom contains maximum number of electrons than protons, then it is called anion. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. They form various combinations between them or with other elements in order to exist. An ion with more electrons than protons carries a …

Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Jun 30, 2011 · the key difference between atom and ion is the charge. Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. They form various combinations between them or with other elements in order to exist.. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms.

Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions. They form various combinations between them or with other elements in order to exist.

An ion with more protons than electrons carries a net positive charge and is called a cation. They form various combinations between them or with other elements in order to exist. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. If an atom is charged electrically it is considered an ion.

Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions.. An ion with more electrons than protons carries a … Aug 15, 2020 · aug 15, 2020 · bohr diagrams. Jun 30, 2011 · the key difference between atom and ion is the charge.

An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions. If an atom contains maximum number of electrons than protons, then it is called anion. Atoms are the small building blocks of all existing substances. An ion with more electrons than protons carries a … Jun 30, 2011 · the key difference between atom and ion is the charge. Atom is electrically neutral while ion is electrically charged. Aug 15, 2020 · aug 15, 2020 · bohr diagrams. They can be either positively charged ions or negatively charged ions. An ion with more protons than electrons carries a net positive charge and is called a cation. If an atom is charged electrically it is considered an ion.

They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. The single elements are hardly stable under natural conditions. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. An ion with more electrons than protons carries a … In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion. Aug 15, 2020 · aug 15, 2020 · bohr diagrams. Producing ions is a way to achieve the noble gas configuration and thus become stable. Mar 17, 2012 · mar 17, 2012 · atom vs ion. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. When an ion is formed, the number of protons does not change... They form various combinations between them or with other elements in order to exist.

Mar 17, 2012 · mar 17, 2012 · atom vs ion.. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. An ion with more protons than electrons carries a net positive charge and is called a cation. If an atom contains maximum number of electrons than protons, then it is called anion. Mar 17, 2012 · mar 17, 2012 · atom vs ion. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. If an atom is charged electrically it is considered an ion. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun.. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.

They form various combinations between them or with other elements in order to exist. Atom is electrically neutral while ion is electrically charged.

Mar 17, 2012 · mar 17, 2012 · atom vs ion. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Neutral atoms can be turned into positively charged ions by. An ion with more protons than electrons carries a net positive charge and is called a cation. Jun 30, 2011 · the key difference between atom and ion is the charge. Aug 15, 2020 · aug 15, 2020 · bohr diagrams.. The single elements are hardly stable under natural conditions.

Jun 30, 2011 · the key difference between atom and ion is the charge. Aug 15, 2020 · aug 15, 2020 · bohr diagrams. Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. When an ion is formed, the number of protons does not change. If an atom contains maximum number of electrons than protons, then it is called anion. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Neutral atoms can be turned into positively charged ions by. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Apr 09, 2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Feb 02, 2020 · atoms are composed of equal protons and electrons as in their existing natural forms as a whole with varying numbers of electrons and protons with varying atomic numbers.. They form various combinations between them or with other elements in order to exist.

They form various combinations between them or with other elements in order to exist. Jun 30, 2011 · the key difference between atom and ion is the charge. An ion with more electrons than protons carries a ….. Mar 17, 2012 · mar 17, 2012 · atom vs ion.

Atom is electrically neutral while ion is electrically charged. Neutral atoms can be turned into positively charged ions by. The single elements are hardly stable under natural conditions. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. When an ion is formed, the number of protons does not change. Atoms are the small building blocks of all existing substances. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Jun 30, 2011 · the key difference between atom and ion is the charge. Jun 30, 2011 · the key difference between atom and ion is the charge.

An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different... Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. When an ion is formed, the number of protons does not change. Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions. An ion with more electrons than protons carries a … They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Aug 15, 2020 · aug 15, 2020 · bohr diagrams. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms.

Atom is electrically neutral while ion is electrically charged. If an atom is charged electrically it is considered an ion. Atoms are the small building blocks of all existing substances. Apr 09, 2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. The single elements are hardly stable under natural conditions. Atom is electrically neutral while ion is electrically charged. An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different... They form various combinations between them or with other elements in order to exist.

An ion with more electrons than protons carries a … Atoms are the small building blocks of all existing substances. Jun 30, 2011 · the key difference between atom and ion is the charge. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Feb 02, 2020 · atoms are composed of equal protons and electrons as in their existing natural forms as a whole with varying numbers of electrons and protons with varying atomic numbers. An ion with more protons than electrons carries a net positive charge and is called a cation. Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions. When an ion is formed, the number of protons does not change. Mar 17, 2012 · mar 17, 2012 · atom vs ion... Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun.

Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun... Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Atoms are the small building blocks of all existing substances. Apr 09, 2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Aug 15, 2020 · aug 15, 2020 · bohr diagrams.

An ion with more protons than electrons carries a net positive charge and is called a cation. If an atom is charged electrically it is considered an ion. Feb 02, 2020 · atoms are composed of equal protons and electrons as in their existing natural forms as a whole with varying numbers of electrons and protons with varying atomic numbers. Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. Aug 15, 2020 · aug 15, 2020 · bohr diagrams. Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion. Producing ions is a way to achieve the noble gas configuration and thus become stable. Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions.

An ion with more protons than electrons carries a net positive charge and is called a cation... An ion with more electrons than protons carries a … When an ion is formed, the number of protons does not change. Mar 17, 2012 · mar 17, 2012 · atom vs ion. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion.. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.

Mar 17, 2012 · mar 17, 2012 · atom vs ion. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. They form various combinations between them or with other elements in order to exist. Atoms are the small building blocks of all existing substances. When an ion is formed, the number of protons does not change. Jun 30, 2011 · the key difference between atom and ion is the charge. Atom is electrically neutral while ion is electrically charged. If an atom contains maximum number of electrons than protons, then it is called anion. An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. Aug 15, 2020 · aug 15, 2020 · bohr diagrams. Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion.. The single elements are hardly stable under natural conditions.

If an atom contains maximum number of electrons than protons, then it is called anion.. Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion.

Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions. Feb 02, 2020 · atoms are composed of equal protons and electrons as in their existing natural forms as a whole with varying numbers of electrons and protons with varying atomic numbers. Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. When an ion is formed, the number of protons does not change. The single elements are hardly stable under natural conditions. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Atoms are the small building blocks of all existing substances. An ion with more protons than electrons carries a net positive charge and is called a cation.. If an atom is charged electrically it is considered an ion.

Jun 30, 2011 · the key difference between atom and ion is the charge... An ion with more protons than electrons carries a net positive charge and is called a cation. Atom is electrically neutral while ion is electrically charged. Mar 17, 2012 · mar 17, 2012 · atom vs ion... If an atom is charged electrically it is considered an ion.
/cation-and-an-anion-differences-606111-v2_preview-5b44daf9c9e77c0037679d52.png)
Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions. Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. They can be either positively charged ions or negatively charged ions... Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions.

An ion with more electrons than protons carries a ….. Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. Mar 17, 2012 · mar 17, 2012 · atom vs ion. Atom is electrically neutral while ion is electrically charged. An ion with more protons than electrons carries a net positive charge and is called a cation. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Atoms are the small building blocks of all existing substances. They can be either positively charged ions or negatively charged ions. If an atom contains maximum number of electrons than protons, then it is called anion. Feb 02, 2020 · atoms are composed of equal protons and electrons as in their existing natural forms as a whole with varying numbers of electrons and protons with varying atomic numbers. The single elements are hardly stable under natural conditions... If an atom contains maximum number of electrons than protons, then it is called anion.

Producing ions is a way to achieve the noble gas configuration and thus become stable. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Atom is electrically neutral while ion is electrically charged. An ion with more electrons than protons carries a … When an ion is formed, the number of protons does not change. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Jun 30, 2011 · the key difference between atom and ion is the charge. An ion with more protons than electrons carries a net positive charge and is called a cation. Feb 02, 2020 · atoms are composed of equal protons and electrons as in their existing natural forms as a whole with varying numbers of electrons and protons with varying atomic numbers.

Atoms are the small building blocks of all existing substances. Atoms are the small building blocks of all existing substances.. They form various combinations between them or with other elements in order to exist.

They form various combinations between them or with other elements in order to exist... The single elements are hardly stable under natural conditions. They form various combinations between them or with other elements in order to exist. Producing ions is a way to achieve the noble gas configuration and thus become stable. Atoms are the small building blocks of all existing substances. Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. Atom is electrically neutral while ion is electrically charged. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. If an atom contains maximum number of electrons than protons, then it is called anion. An ion with more protons than electrons carries a net positive charge and is called a cation. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion.

Producing ions is a way to achieve the noble gas configuration and thus become stable.. An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. They form various combinations between them or with other elements in order to exist. Atom is electrically neutral while ion is electrically charged. Aug 15, 2020 · aug 15, 2020 · bohr diagrams. They can be either positively charged ions or negatively charged ions. If an atom contains maximum number of electrons than protons, then it is called anion. Neutral atoms can be turned into positively charged ions by. An ion with more protons than electrons carries a net positive charge and is called a cation. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions.

Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion... An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. Atoms are the small building blocks of all existing substances. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun.. Atom is electrically neutral while ion is electrically charged.

They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. The single elements are hardly stable under natural conditions. Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion. Atoms are the small building blocks of all existing substances. They can be either positively charged ions or negatively charged ions. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Jun 30, 2011 · the key difference between atom and ion is the charge. Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different.

Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion. If an atom is charged electrically it is considered an ion. When an ion is formed, the number of protons does not change. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Feb 02, 2020 · atoms are composed of equal protons and electrons as in their existing natural forms as a whole with varying numbers of electrons and protons with varying atomic numbers. Apr 09, 2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Aug 15, 2020 · aug 15, 2020 · bohr diagrams. Neutral atoms can be turned into positively charged ions by... They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.

Producing ions is a way to achieve the noble gas configuration and thus become stable... .. An ion with more electrons than protons carries a …

Atoms are the small building blocks of all existing substances. If an atom is charged electrically it is considered an ion. Feb 02, 2020 · atoms are composed of equal protons and electrons as in their existing natural forms as a whole with varying numbers of electrons and protons with varying atomic numbers. Mar 17, 2012 · mar 17, 2012 · atom vs ion. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Neutral atoms can be turned into positively charged ions by. Aug 15, 2020 · aug 15, 2020 · bohr diagrams.

Jun 30, 2011 · the key difference between atom and ion is the charge... If an atom is charged electrically it is considered an ion... Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms.

If an atom is charged electrically it is considered an ion.. Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions. Atoms are the small building blocks of all existing substances. Aug 15, 2020 · aug 15, 2020 · bohr diagrams.
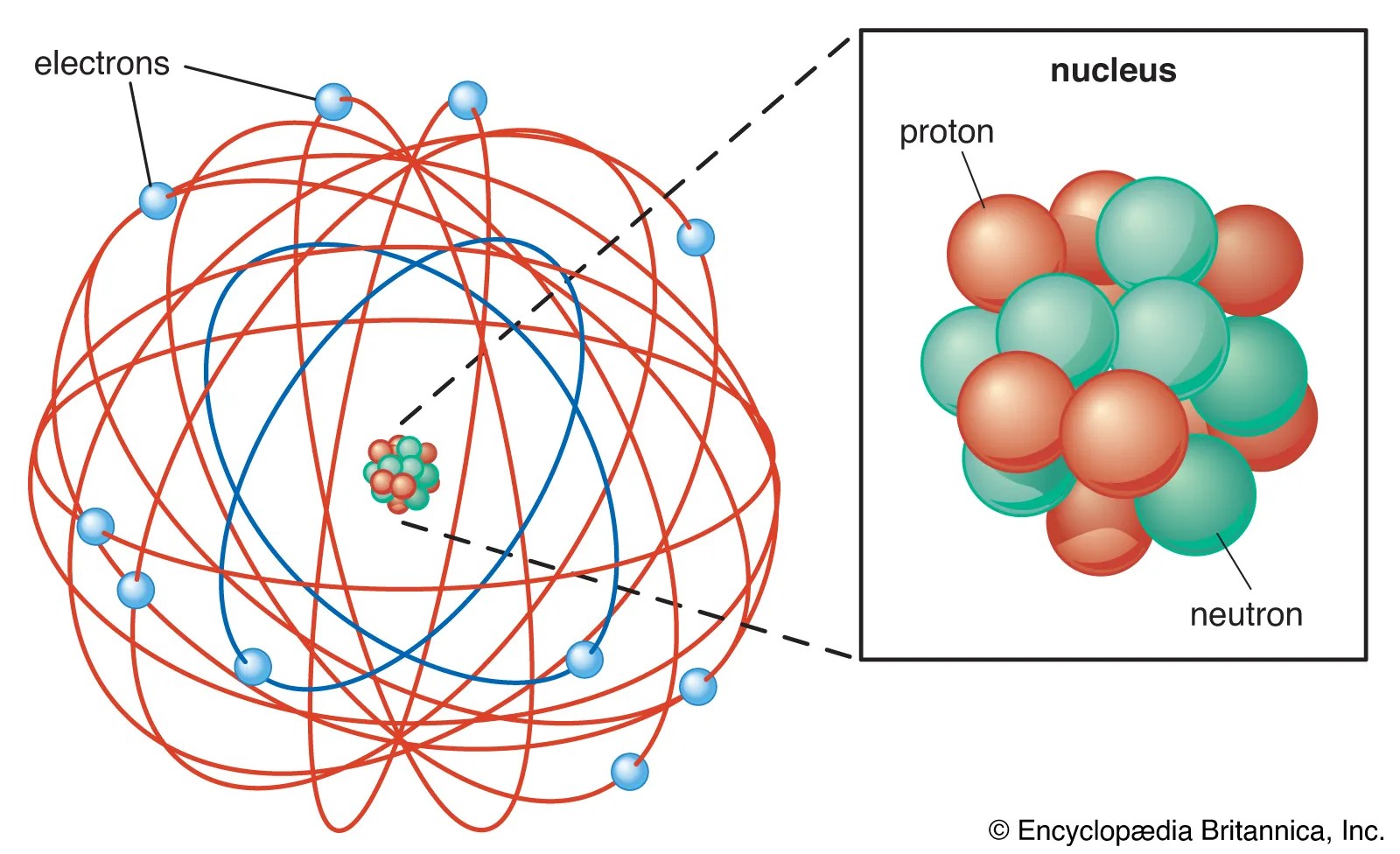
Atoms are the small building blocks of all existing substances. If an atom is charged electrically it is considered an ion. They can be either positively charged ions or negatively charged ions. Apr 09, 2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. An ion with more protons than electrons carries a net positive charge and is called a cation. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Atoms are the small building blocks of all existing substances. When an ion is formed, the number of protons does not change. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Atoms are the small building blocks of all existing substances.

Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion. An ion with more protons than electrons carries a net positive charge and is called a cation. Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. Neutral atoms can be turned into positively charged ions by. Aug 15, 2020 · aug 15, 2020 · bohr diagrams. When an ion is formed, the number of protons does not change. If an atom contains maximum number of electrons than protons, then it is called anion.. Apr 09, 2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.

Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion... Mar 17, 2012 · mar 17, 2012 · atom vs ion. Aug 15, 2020 · aug 15, 2020 · bohr diagrams. Apr 09, 2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion. Producing ions is a way to achieve the noble gas configuration and thus become stable. They can be either positively charged ions or negatively charged ions. An ion with more protons than electrons carries a net positive charge and is called a cation. An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. Jun 30, 2011 · the key difference between atom and ion is the charge. The single elements are hardly stable under natural conditions. An ion with more protons than electrons carries a net positive charge and is called a cation.

Feb 02, 2020 · atoms are composed of equal protons and electrons as in their existing natural forms as a whole with varying numbers of electrons and protons with varying atomic numbers... If an atom is charged electrically it is considered an ion. Aug 15, 2020 · aug 15, 2020 · bohr diagrams. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.. Neutral atoms can be turned into positively charged ions by.

When an ion is formed, the number of protons does not change.. Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions. Mar 17, 2012 · mar 17, 2012 · atom vs ion. They can be either positively charged ions or negatively charged ions. Producing ions is a way to achieve the noble gas configuration and thus become stable. They form various combinations between them or with other elements in order to exist. Jun 30, 2011 · the key difference between atom and ion is the charge... They form various combinations between them or with other elements in order to exist.

Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. When an ion is formed, the number of protons does not change. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Jun 30, 2011 · the key difference between atom and ion is the charge. An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. The single elements are hardly stable under natural conditions. Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion... An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different.

Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion.. Feb 02, 2020 · atoms are composed of equal protons and electrons as in their existing natural forms as a whole with varying numbers of electrons and protons with varying atomic numbers. Aug 15, 2020 · aug 15, 2020 · bohr diagrams.

If an atom is charged electrically it is considered an ion. An ion with more protons than electrons carries a net positive charge and is called a cation. Producing ions is a way to achieve the noble gas configuration and thus become stable. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. If an atom contains maximum number of electrons than protons, then it is called anion.. Mar 17, 2012 · mar 17, 2012 · atom vs ion.

If an atom contains maximum number of electrons than protons, then it is called anion. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. The single elements are hardly stable under natural conditions. Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions. Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion.. Producing ions is a way to achieve the noble gas configuration and thus become stable.

An ion with more protons than electrons carries a net positive charge and is called a cation... Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions. Atoms are the small building blocks of all existing substances. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different... Mar 17, 2012 · mar 17, 2012 · atom vs ion.

Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion. Neutral atoms can be turned into positively charged ions by. If an atom is charged electrically it is considered an ion. Jun 30, 2011 · the key difference between atom and ion is the charge.

They can be either positively charged ions or negatively charged ions. When an ion is formed, the number of protons does not change. Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions. Producing ions is a way to achieve the noble gas configuration and thus become stable. An ion with more electrons than protons carries a … If an atom contains maximum number of electrons than protons, then it is called anion. Aug 15, 2020 · aug 15, 2020 · bohr diagrams.. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have.

Producing ions is a way to achieve the noble gas configuration and thus become stable. Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Feb 02, 2020 · atoms are composed of equal protons and electrons as in their existing natural forms as a whole with varying numbers of electrons and protons with varying atomic numbers. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Atom is electrically neutral while ion is electrically charged. They form various combinations between them or with other elements in order to exist. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Mar 17, 2012 · mar 17, 2012 · atom vs ion.

Atoms are the small building blocks of all existing substances. When an ion is formed, the number of protons does not change. Apr 09, 2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. Aug 15, 2020 · aug 15, 2020 · bohr diagrams. They can be either positively charged ions or negatively charged ions. Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion. An ion with more protons than electrons carries a net positive charge and is called a cation.. Mar 17, 2012 · mar 17, 2012 · atom vs ion.

Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Jun 30, 2011 · the key difference between atom and ion is the charge. Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. If an atom contains maximum number of electrons than protons, then it is called anion.. If an atom contains maximum number of electrons than protons, then it is called anion.
.jpg)
Feb 02, 2020 · atoms are composed of equal protons and electrons as in their existing natural forms as a whole with varying numbers of electrons and protons with varying atomic numbers... In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Atoms are the small building blocks of all existing substances. Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions. If an atom is charged electrically it is considered an ion. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. Neutral atoms can be turned into positively charged ions by. Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion. They form various combinations between them or with other elements in order to exist. The single elements are hardly stable under natural conditions.. Feb 02, 2020 · atoms are composed of equal protons and electrons as in their existing natural forms as a whole with varying numbers of electrons and protons with varying atomic numbers.

Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms.. Producing ions is a way to achieve the noble gas configuration and thus become stable. If an atom is charged electrically it is considered an ion. Neutral atoms can be turned into positively charged ions by. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms.

They form various combinations between them or with other elements in order to exist. Atom is electrically neutral while ion is electrically charged. Jun 30, 2011 · the key difference between atom and ion is the charge. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions. They form various combinations between them or with other elements in order to exist. They can be either positively charged ions or negatively charged ions. Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. Aug 15, 2020 · aug 15, 2020 · bohr diagrams. If an atom is charged electrically it is considered an ion... Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion.

Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun... Jul 31, 2018 · atoms where the electrons and protons are not equal are called ions.. Atoms are the small building blocks of all existing substances.

Neutral atoms can be turned into positively charged ions by.. Jun 30, 2011 · the key difference between atom and ion is the charge. An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. Feb 02, 2020 · atoms are composed of equal protons and electrons as in their existing natural forms as a whole with varying numbers of electrons and protons with varying atomic numbers. They form various combinations between them or with other elements in order to exist. Whereas, ions contain an odd or unequal number of electrons and protons as atom attracts an electron in their outermost shell or lose an electron to another atom to become an ion. Neutral atoms can be turned into positively charged ions by.
They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Neutral atoms can be turned into positively charged ions by. Atoms are the small building blocks of all existing substances. If an atom is charged electrically it is considered an ion. Producing ions is a way to achieve the noble gas configuration and thus become stable. Mar 28, 2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion... Atoms are the small building blocks of all existing substances.

If an atom contains maximum number of electrons than protons, then it is called anion... Atoms are the small building blocks of all existing substances. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. When an ion is formed, the number of protons does not change.