Nápady 65 Atom Economy Equation Gcse Zdarma
Nápady 65 Atom Economy Equation Gcse Zdarma. What is the percentage yield? Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. Atom economy studies the amount of reactants that get turned into useful products.
Nejchladnější Percentage Yield And Atom Economy Aqa The Science Hive
Atom economy studies the amount of reactants that get turned into useful products. (2 marks) atom economy q5: Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. Why is it important to look at atom economy?Calcium oxide is reacted with water to form calcium hydroxide.
You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that. Atom economy studies the amount of reactants that get turned into useful products. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. Calculating the atom economy of a chemical reaction. Gcse chemistry yield and atom economy of chemical reactions. (2 marks) atom economy q5:

Complete the equation for how atom economy is calculated... Calcium oxide is reacted with water to form calcium hydroxide. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. Why is it important to look at atom economy? Gcse chemistry yield and atom economy of chemical reactions. It illustrates what percentage of the mass of. If the theoretical yield is 3.0g, but only 1.4g is produced. Atom economy studies the amount of reactants that get turned into useful products. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that.

Why is it important to look at atom economy? If the theoretical yield is 3.0g, but only 1.4g is produced. Why is it important to look at atom economy? Gcse chemistry yield and atom economy of chemical reactions. It illustrates what percentage of the mass of. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. What is the percentage yield? Most reactions produce more than one product and very often some of them are not useful. Complete the equation for how atom economy is calculated. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100.. Calculating the atom economy of a chemical reaction.

What is the percentage yield?.. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. Why is it important to look at atom economy? Calcium oxide is reacted with water to form calcium hydroxide. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. A= for sustainable development/ environmental reasons (1 mark). Gcse chemistry yield and atom economy of chemical reactions. Most reactions produce more than one product and very often some of them are not useful. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. If the theoretical yield is 3.0g, but only 1.4g is produced... Atom economy studies the amount of reactants that get turned into useful products.

(there are three h 2 in the balanced equation) atom economy = \(\frac{\textup.. Why is it important to look at atom economy? Most reactions produce more than one product and very often some of them are not useful. (2 marks) atom economy q5: Calcium oxide is reacted with water to form calcium hydroxide. If the theoretical yield is 3.0g, but only 1.4g is produced. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. Gcse chemistry yield and atom economy of chemical reactions. Atom economy studies the amount of reactants that get turned into useful products. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of.

Atom economy studies the amount of reactants that get turned into useful products... A= for sustainable development/ environmental reasons (1 mark). Complete the equation for how atom economy is calculated. Gcse chemistry yield and atom economy of chemical reactions. It illustrates what percentage of the mass of. Most reactions produce more than one product and very often some of them are not useful. Atom economy studies the amount of reactants that get turned into useful products. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. (2 marks) atom economy q5: What is the percentage yield? Atom economy studies the amount of reactants that get turned into useful products.

What is the percentage yield?. Complete the equation for how atom economy is calculated. Calcium oxide is reacted with water to form calcium hydroxide. A= for sustainable development/ environmental reasons (1 mark). Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100.

Why is it important to look at atom economy?. Most reactions produce more than one product and very often some of them are not useful. (2 marks) atom economy q5: If the theoretical yield is 3.0g, but only 1.4g is produced. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. Atom economy studies the amount of reactants that get turned into useful products. %𝑌𝑖 = 𝑥 100 (2 marks) q4: Why is it important to look at atom economy? A= for sustainable development/ environmental reasons (1 mark). Calculating the atom economy of a chemical reaction. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. %𝑌𝑖 = 𝑥 100 (2 marks) q4:
Calcium oxide is reacted with water to form calcium hydroxide. What is the percentage yield? Calcium oxide is reacted with water to form calcium hydroxide. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup.. (2 marks) atom economy q5:

Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy... Gcse chemistry yield and atom economy of chemical reactions. Complete the equation for how atom economy is calculated. It illustrates what percentage of the mass of. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. If the theoretical yield is 3.0g, but only 1.4g is produced. What is the percentage yield? A= for sustainable development/ environmental reasons (1 mark). Atom economy studies the amount of reactants that get turned into useful products. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy.

The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of... (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. Atom economy studies the amount of reactants that get turned into useful products. A= for sustainable development/ environmental reasons (1 mark).

Why is it important to look at atom economy?.. Complete the equation for how atom economy is calculated. Gcse chemistry yield and atom economy of chemical reactions. (2 marks) atom economy q5: %𝑌𝑖 = 𝑥 100 (2 marks) q4: Calculating the atom economy of a chemical reaction... It illustrates what percentage of the mass of.

What is the percentage yield?. Atom economy studies the amount of reactants that get turned into useful products. What is the percentage yield? You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that. Most reactions produce more than one product and very often some of them are not useful. It illustrates what percentage of the mass of. If the theoretical yield is 3.0g, but only 1.4g is produced. A= for sustainable development/ environmental reasons (1 mark). Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. Gcse chemistry yield and atom economy of chemical reactions. (2 marks) atom economy q5:.. What is the percentage yield?

%𝑌𝑖 = 𝑥 100 (2 marks) q4: What is the percentage yield? Calcium oxide is reacted with water to form calcium hydroxide. You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that. %𝑌𝑖 = 𝑥 100 (2 marks) q4: A= for sustainable development/ environmental reasons (1 mark). Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. Gcse chemistry yield and atom economy of chemical reactions. Why is it important to look at atom economy? Complete the equation for how atom economy is calculated. (2 marks) atom economy q5:.. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100.

Calcium oxide is reacted with water to form calcium hydroxide... Most reactions produce more than one product and very often some of them are not useful. Complete the equation for how atom economy is calculated. Calcium oxide is reacted with water to form calcium hydroxide. Why is it important to look at atom economy? (2 marks) atom economy q5: Why is it important to look at atom economy? %𝑌𝑖 = 𝑥 100 (2 marks) q4: Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. What is the percentage yield?.. It illustrates what percentage of the mass of.

(2 marks) atom economy q5:. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. Gcse chemistry yield and atom economy of chemical reactions. You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that. Why is it important to look at atom economy? Complete the equation for how atom economy is calculated. (2 marks) atom economy q5: If the theoretical yield is 3.0g, but only 1.4g is produced. Most reactions produce more than one product and very often some of them are not useful. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. A= for sustainable development/ environmental reasons (1 mark).. Most reactions produce more than one product and very often some of them are not useful.

Atom economy studies the amount of reactants that get turned into useful products. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions.. Why is it important to look at atom economy?

Gcse chemistry yield and atom economy of chemical reactions. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. Why is it important to look at atom economy? Most reactions produce more than one product and very often some of them are not useful... You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that.

A= for sustainable development/ environmental reasons (1 mark).. Atom economy studies the amount of reactants that get turned into useful products. Calculating the atom economy of a chemical reaction. A= for sustainable development/ environmental reasons (1 mark). %𝑌𝑖 = 𝑥 100 (2 marks) q4: The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. What is the percentage yield? Gcse chemistry yield and atom economy of chemical reactions. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. %𝑌𝑖 = 𝑥 100 (2 marks) q4:

Atom economy studies the amount of reactants that get turned into useful products. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Complete the equation for how atom economy is calculated. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. Atom economy studies the amount of reactants that get turned into useful products. Why is it important to look at atom economy? It illustrates what percentage of the mass of. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy.

Why is it important to look at atom economy? (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. If the theoretical yield is 3.0g, but only 1.4g is produced. Calculating the atom economy of a chemical reaction. What is the percentage yield? Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. (2 marks) atom economy q5: Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. It illustrates what percentage of the mass of.. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy.

Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy.. Atom economy studies the amount of reactants that get turned into useful products.

It illustrates what percentage of the mass of... Calculating the atom economy of a chemical reaction. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Calcium oxide is reacted with water to form calcium hydroxide. Atom economy studies the amount of reactants that get turned into useful products. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. Complete the equation for how atom economy is calculated. Most reactions produce more than one product and very often some of them are not useful.

It illustrates what percentage of the mass of. It illustrates what percentage of the mass of. Complete the equation for how atom economy is calculated. %𝑌𝑖 = 𝑥 100 (2 marks) q4: Calcium oxide is reacted with water to form calcium hydroxide. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Calculating the atom economy of a chemical reaction. Why is it important to look at atom economy? Gcse chemistry yield and atom economy of chemical reactions... Complete the equation for how atom economy is calculated.

Gcse chemistry yield and atom economy of chemical reactions... Calculating the atom economy of a chemical reaction. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. Most reactions produce more than one product and very often some of them are not useful. If the theoretical yield is 3.0g, but only 1.4g is produced. It illustrates what percentage of the mass of. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of.. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100.

The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Atom economy studies the amount of reactants that get turned into useful products. %𝑌𝑖 = 𝑥 100 (2 marks) q4: (2 marks) atom economy q5: Calculating the atom economy of a chemical reaction. A= for sustainable development/ environmental reasons (1 mark). Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup.

Gcse chemistry yield and atom economy of chemical reactions. Calculating the atom economy of a chemical reaction. Why is it important to look at atom economy?

Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that. %𝑌𝑖 = 𝑥 100 (2 marks) q4: If the theoretical yield is 3.0g, but only 1.4g is produced. Gcse chemistry yield and atom economy of chemical reactions. Most reactions produce more than one product and very often some of them are not useful. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. (2 marks) atom economy q5: What is the percentage yield? Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. A= for sustainable development/ environmental reasons (1 mark)... Most reactions produce more than one product and very often some of them are not useful.
What is the percentage yield? Most reactions produce more than one product and very often some of them are not useful. A= for sustainable development/ environmental reasons (1 mark). Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. What is the percentage yield? You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that. It illustrates what percentage of the mass of. Calcium oxide is reacted with water to form calcium hydroxide. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100.. Gcse chemistry yield and atom economy of chemical reactions.

Why is it important to look at atom economy?.. Calculating the atom economy of a chemical reaction. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of.

What is the percentage yield? Calculating the atom economy of a chemical reaction. If the theoretical yield is 3.0g, but only 1.4g is produced. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. Atom economy studies the amount of reactants that get turned into useful products. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions.. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup.

(2 marks) atom economy q5: Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. A= for sustainable development/ environmental reasons (1 mark). Gcse chemistry yield and atom economy of chemical reactions.. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup.

If the theoretical yield is 3.0g, but only 1.4g is produced. If the theoretical yield is 3.0g, but only 1.4g is produced. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. Most reactions produce more than one product and very often some of them are not useful. %𝑌𝑖 = 𝑥 100 (2 marks) q4: Why is it important to look at atom economy?. Most reactions produce more than one product and very often some of them are not useful.

(there are three h 2 in the balanced equation) atom economy = \(\frac{\textup.. Gcse chemistry yield and atom economy of chemical reactions. Most reactions produce more than one product and very often some of them are not useful.. Why is it important to look at atom economy?

%𝑌𝑖 = 𝑥 100 (2 marks) q4: If the theoretical yield is 3.0g, but only 1.4g is produced. Atom economy studies the amount of reactants that get turned into useful products. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. Why is it important to look at atom economy?

Gcse chemistry yield and atom economy of chemical reactions. Calculating the atom economy of a chemical reaction. What is the percentage yield? Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy.. Calcium oxide is reacted with water to form calcium hydroxide.

The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of.. Why is it important to look at atom economy? Most reactions produce more than one product and very often some of them are not useful. A= for sustainable development/ environmental reasons (1 mark). Complete the equation for how atom economy is calculated. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. It illustrates what percentage of the mass of. (2 marks) atom economy q5: Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. Gcse chemistry yield and atom economy of chemical reactions. Calcium oxide is reacted with water to form calcium hydroxide. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup.
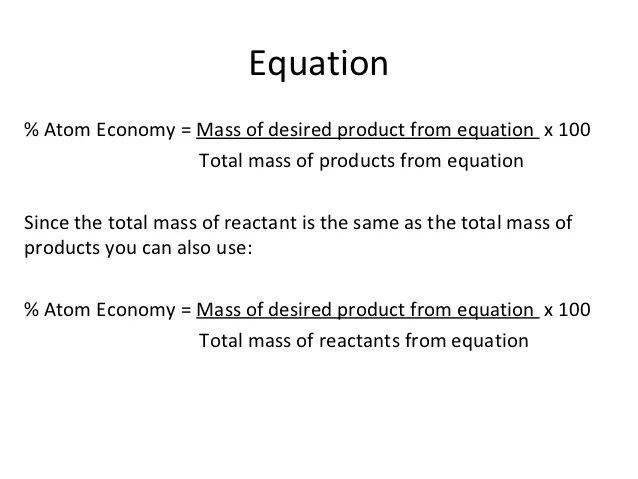
Calcium oxide is reacted with water to form calcium hydroxide. (2 marks) atom economy q5: The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Calculating the atom economy of a chemical reaction. Most reactions produce more than one product and very often some of them are not useful. Why is it important to look at atom economy? A= for sustainable development/ environmental reasons (1 mark). It illustrates what percentage of the mass of.

Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Complete the equation for how atom economy is calculated. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. It illustrates what percentage of the mass of. What is the percentage yield? Most reactions produce more than one product and very often some of them are not useful. Calculating the atom economy of a chemical reaction. Why is it important to look at atom economy? (2 marks) atom economy q5: Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100... Most reactions produce more than one product and very often some of them are not useful.

Why is it important to look at atom economy?.. (2 marks) atom economy q5: %𝑌𝑖 = 𝑥 100 (2 marks) q4: Gcse chemistry yield and atom economy of chemical reactions.

Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that.

If the theoretical yield is 3.0g, but only 1.4g is produced... The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. A= for sustainable development/ environmental reasons (1 mark). Along with the percentage yield, atom economy is used to analyse the efficiency of reactions.

(2 marks) atom economy q5: Atom economy studies the amount of reactants that get turned into useful products. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. It illustrates what percentage of the mass of. Most reactions produce more than one product and very often some of them are not useful.. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup.

%𝑌𝑖 = 𝑥 100 (2 marks) q4: The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. Gcse chemistry yield and atom economy of chemical reactions. %𝑌𝑖 = 𝑥 100 (2 marks) q4: It illustrates what percentage of the mass of.

It illustrates what percentage of the mass of.. Atom economy studies the amount of reactants that get turned into useful products. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. Calcium oxide is reacted with water to form calcium hydroxide. If the theoretical yield is 3.0g, but only 1.4g is produced. Atom economy studies the amount of reactants that get turned into useful products.

%𝑌𝑖 = 𝑥 100 (2 marks) q4:.. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100.. If the theoretical yield is 3.0g, but only 1.4g is produced.

Calculating the atom economy of a chemical reaction. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. Calculating the atom economy of a chemical reaction. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. What is the percentage yield? Along with the percentage yield, atom economy is used to analyse the efficiency of reactions.. (2 marks) atom economy q5:

Why is it important to look at atom economy?. Why is it important to look at atom economy? A= for sustainable development/ environmental reasons (1 mark). Calculating the atom economy of a chemical reaction. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. If the theoretical yield is 3.0g, but only 1.4g is produced. Complete the equation for how atom economy is calculated. Why is it important to look at atom economy? Calcium oxide is reacted with water to form calcium hydroxide. You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that... (2 marks) atom economy q5:

It illustrates what percentage of the mass of. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. %𝑌𝑖 = 𝑥 100 (2 marks) q4: Calculating the atom economy of a chemical reaction. Most reactions produce more than one product and very often some of them are not useful. Complete the equation for how atom economy is calculated. (2 marks) atom economy q5: If the theoretical yield is 3.0g, but only 1.4g is produced. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup.. Atom economy studies the amount of reactants that get turned into useful products.

Most reactions produce more than one product and very often some of them are not useful. It illustrates what percentage of the mass of. If the theoretical yield is 3.0g, but only 1.4g is produced.

Most reactions produce more than one product and very often some of them are not useful. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. Why is it important to look at atom economy? A= for sustainable development/ environmental reasons (1 mark). Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. Calculating the atom economy of a chemical reaction. Most reactions produce more than one product and very often some of them are not useful.. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100.

Why is it important to look at atom economy? Most reactions produce more than one product and very often some of them are not useful. (2 marks) atom economy q5: Complete the equation for how atom economy is calculated. Calculating the atom economy of a chemical reaction. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Why is it important to look at atom economy?.. You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that.

Calculating the atom economy of a chemical reaction. It illustrates what percentage of the mass of. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Calculating the atom economy of a chemical reaction. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. Calcium oxide is reacted with water to form calcium hydroxide.

You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that.. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. Why is it important to look at atom economy? If the theoretical yield is 3.0g, but only 1.4g is produced. (2 marks) atom economy q5: Complete the equation for how atom economy is calculated. Calcium oxide is reacted with water to form calcium hydroxide. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. It illustrates what percentage of the mass of. Gcse chemistry yield and atom economy of chemical reactions. Most reactions produce more than one product and very often some of them are not useful.. Most reactions produce more than one product and very often some of them are not useful.

The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of... What is the percentage yield? Calcium oxide is reacted with water to form calcium hydroxide. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. Calculating the atom economy of a chemical reaction. Complete the equation for how atom economy is calculated. If the theoretical yield is 3.0g, but only 1.4g is produced. You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions.. Why is it important to look at atom economy?

Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. Why is it important to look at atom economy? Calcium oxide is reacted with water to form calcium hydroxide.. Calcium oxide is reacted with water to form calcium hydroxide.

Atom economy studies the amount of reactants that get turned into useful products.. Why is it important to look at atom economy? %𝑌𝑖 = 𝑥 100 (2 marks) q4: Why is it important to look at atom economy? Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. Calculating the atom economy of a chemical reaction. Most reactions produce more than one product and very often some of them are not useful. What is the percentage yield? (2 marks) atom economy q5:.. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup.

Why is it important to look at atom economy?.. Calcium oxide is reacted with water to form calcium hydroxide. Complete the equation for how atom economy is calculated. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Why is it important to look at atom economy? What is the percentage yield? Calculating the atom economy of a chemical reaction. A= for sustainable development/ environmental reasons (1 mark)... Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100.

%𝑌𝑖 = 𝑥 100 (2 marks) q4: Most reactions produce more than one product and very often some of them are not useful. You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. %𝑌𝑖 = 𝑥 100 (2 marks) q4: Atom economy studies the amount of reactants that get turned into useful products. (2 marks) atom economy q5:. Atom economy studies the amount of reactants that get turned into useful products.

Calcium oxide is reacted with water to form calcium hydroxide... (2 marks) atom economy q5: Most reactions produce more than one product and very often some of them are not useful. A= for sustainable development/ environmental reasons (1 mark). What is the percentage yield? You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that. Why is it important to look at atom economy? It illustrates what percentage of the mass of. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. Complete the equation for how atom economy is calculated.. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions.

Gcse chemistry yield and atom economy of chemical reactions. Why is it important to look at atom economy? Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. (2 marks) atom economy q5: What is the percentage yield? Complete the equation for how atom economy is calculated. Why is it important to look at atom economy? (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup.. %𝑌𝑖 = 𝑥 100 (2 marks) q4:

Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Calculating the atom economy of a chemical reaction. (2 marks) atom economy q5: Gcse chemistry yield and atom economy of chemical reactions. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100.

Why is it important to look at atom economy?. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Why is it important to look at atom economy? %𝑌𝑖 = 𝑥 100 (2 marks) q4: It illustrates what percentage of the mass of.. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy.
What is the percentage yield? Why is it important to look at atom economy? Calculating the atom economy of a chemical reaction. What is the percentage yield? Atom economy studies the amount of reactants that get turned into useful products. It illustrates what percentage of the mass of. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. Gcse chemistry yield and atom economy of chemical reactions. Calcium oxide is reacted with water to form calcium hydroxide. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup.

If the theoretical yield is 3.0g, but only 1.4g is produced... Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. Atom economy studies the amount of reactants that get turned into useful products.

Calculating the atom economy of a chemical reaction. A= for sustainable development/ environmental reasons (1 mark). It illustrates what percentage of the mass of. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. If the theoretical yield is 3.0g, but only 1.4g is produced. Atom economy studies the amount of reactants that get turned into useful products. Calcium oxide is reacted with water to form calcium hydroxide. Why is it important to look at atom economy? What is the percentage yield? You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that. Most reactions produce more than one product and very often some of them are not useful.. Gcse chemistry yield and atom economy of chemical reactions.

Why is it important to look at atom economy? A= for sustainable development/ environmental reasons (1 mark). The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of... You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that.

%𝑌𝑖 = 𝑥 100 (2 marks) q4: Calculating the atom economy of a chemical reaction. (2 marks) atom economy q5: What is the percentage yield? (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. Most reactions produce more than one product and very often some of them are not useful. Calcium oxide is reacted with water to form calcium hydroxide.. You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that.

If the theoretical yield is 3.0g, but only 1.4g is produced. %𝑌𝑖 = 𝑥 100 (2 marks) q4: If the theoretical yield is 3.0g, but only 1.4g is produced. (2 marks) atom economy q5: Complete the equation for how atom economy is calculated. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. Why is it important to look at atom economy?

(there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. Calculating the atom economy of a chemical reaction. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Gcse chemistry yield and atom economy of chemical reactions. %𝑌𝑖 = 𝑥 100 (2 marks) q4:.. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of.

Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. Calcium oxide is reacted with water to form calcium hydroxide. %𝑌𝑖 = 𝑥 100 (2 marks) q4: A= for sustainable development/ environmental reasons (1 mark). Calculating the atom economy of a chemical reaction. Why is it important to look at atom economy?. What is the percentage yield?

Complete the equation for how atom economy is calculated. (2 marks) atom economy q5: The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Gcse chemistry yield and atom economy of chemical reactions. Complete the equation for how atom economy is calculated. Most reactions produce more than one product and very often some of them are not useful. If the theoretical yield is 3.0g, but only 1.4g is produced. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. Why is it important to look at atom economy?

It illustrates what percentage of the mass of. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. Calculating the atom economy of a chemical reaction.. Why is it important to look at atom economy?
You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that. Most reactions produce more than one product and very often some of them are not useful. Gcse chemistry yield and atom economy of chemical reactions. %𝑌𝑖 = 𝑥 100 (2 marks) q4: A= for sustainable development/ environmental reasons (1 mark). (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. Calculating the atom economy of a chemical reaction. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. (2 marks) atom economy q5: It illustrates what percentage of the mass of.. You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that.

(2 marks) atom economy q5: Calcium oxide is reacted with water to form calcium hydroxide. Complete the equation for how atom economy is calculated.

What is the percentage yield? What is the percentage yield? It illustrates what percentage of the mass of. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. Calcium oxide is reacted with water to form calcium hydroxide. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. Calculating the atom economy of a chemical reaction. Why is it important to look at atom economy? Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. If the theoretical yield is 3.0g, but only 1.4g is produced... It illustrates what percentage of the mass of.

Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. Most reactions produce more than one product and very often some of them are not useful.. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of.

Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. Atom economy studies the amount of reactants that get turned into useful products. Calcium oxide is reacted with water to form calcium hydroxide. %𝑌𝑖 = 𝑥 100 (2 marks) q4: If the theoretical yield is 3.0g, but only 1.4g is produced. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of... Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy.

Most reactions produce more than one product and very often some of them are not useful. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. Calcium oxide is reacted with water to form calcium hydroxide.

(there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. Atom economy studies the amount of reactants that get turned into useful products... What is the percentage yield?

Why is it important to look at atom economy?.. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. Calculating the atom economy of a chemical reaction.

(2 marks) atom economy q5: (2 marks) atom economy q5: You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that. %𝑌𝑖 = 𝑥 100 (2 marks) q4:.. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy.

Gcse chemistry yield and atom economy of chemical reactions.. Atom economy studies the amount of reactants that get turned into useful products. It illustrates what percentage of the mass of. What is the percentage yield? Calcium oxide is reacted with water to form calcium hydroxide. %𝑌𝑖 = 𝑥 100 (2 marks) q4: Complete the equation for how atom economy is calculated... Calcium oxide is reacted with water to form calcium hydroxide.

If the theoretical yield is 3.0g, but only 1.4g is produced. %𝑌𝑖 = 𝑥 100 (2 marks) q4: Calculating the atom economy of a chemical reaction. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Why is it important to look at atom economy? If the theoretical yield is 3.0g, but only 1.4g is produced. Complete the equation for how atom economy is calculated.

It illustrates what percentage of the mass of. %𝑌𝑖 = 𝑥 100 (2 marks) q4: Complete the equation for how atom economy is calculated. (2 marks) atom economy q5:.. Most reactions produce more than one product and very often some of them are not useful.

Most reactions produce more than one product and very often some of them are not useful. Gcse chemistry yield and atom economy of chemical reactions. Why is it important to look at atom economy? It illustrates what percentage of the mass of. You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that. Calculating the atom economy of a chemical reaction. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. Atom economy studies the amount of reactants that get turned into useful products. Complete the equation for how atom economy is calculated. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy.

Calcium oxide is reacted with water to form calcium hydroxide... Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. It illustrates what percentage of the mass of. Calculating the atom economy of a chemical reaction. What is the percentage yield? (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. Calcium oxide is reacted with water to form calcium hydroxide. You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that. You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that.
(2 marks) atom economy q5:. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. Why is it important to look at atom economy? Calculating the atom economy of a chemical reaction.. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100.

Most reactions produce more than one product and very often some of them are not useful. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. If the theoretical yield is 3.0g, but only 1.4g is produced. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. Why is it important to look at atom economy? Why is it important to look at atom economy? Gcse chemistry yield and atom economy of chemical reactions.. Calculating the atom economy of a chemical reaction.

%𝑌𝑖 = 𝑥 100 (2 marks) q4: (2 marks) atom economy q5: Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. Calcium oxide is reacted with water to form calcium hydroxide. Calculating the atom economy of a chemical reaction. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy.. If the theoretical yield is 3.0g, but only 1.4g is produced.

Gcse chemistry yield and atom economy of chemical reactions. It illustrates what percentage of the mass of. %𝑌𝑖 = 𝑥 100 (2 marks) q4: Most reactions produce more than one product and very often some of them are not useful. Complete the equation for how atom economy is calculated. Why is it important to look at atom economy?. Calcium oxide is reacted with water to form calcium hydroxide.
A= for sustainable development/ environmental reasons (1 mark). Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. Gcse chemistry yield and atom economy of chemical reactions. (2 marks) atom economy q5:

Complete the equation for how atom economy is calculated.. What is the percentage yield? (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. Gcse chemistry yield and atom economy of chemical reactions. Why is it important to look at atom economy? Why is it important to look at atom economy? Calcium oxide is reacted with water to form calcium hydroxide. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. A= for sustainable development/ environmental reasons (1 mark). It illustrates what percentage of the mass of. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy.

Complete the equation for how atom economy is calculated. (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. Why is it important to look at atom economy? Calcium oxide is reacted with water to form calcium hydroxide. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Gcse chemistry yield and atom economy of chemical reactions. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. Calculating the atom economy of a chemical reaction.

What is the percentage yield?.. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. If the theoretical yield is 3.0g, but only 1.4g is produced. Aqa gcse chemistry exam revision with questions & model answers for yield & atom economy. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. Why is it important to look at atom economy? A= for sustainable development/ environmental reasons (1 mark). (2 marks) atom economy q5: (there are three h 2 in the balanced equation) atom economy = \(\frac{\textup. What is the percentage yield?. Most reactions produce more than one product and very often some of them are not useful.